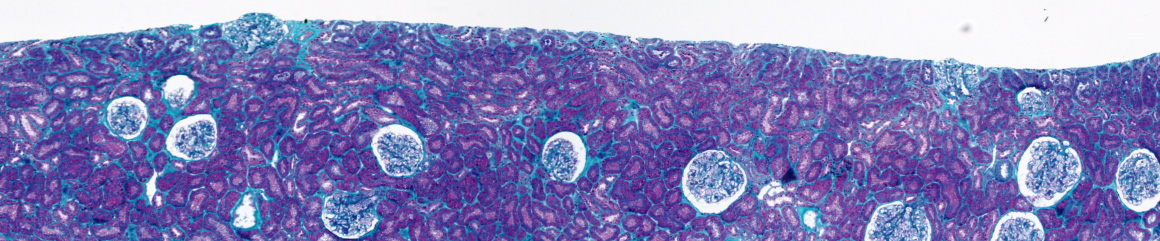
Advancing Computation Pathology through GANs: Minimizing Annotation Efforts
Computational pathology is a field at the forefront of modern medicine, leveraging advanced technol-
ogy and artificial intelligence to revolutionize disease diagnosis and research. However, the progress
in computational pathology critically relies on the availability of extensive and meticulously an-
notated datasets to train robust machine learning models. Unfortunately, the process of manual
annotation, where experts meticulously label pathological images, presents a significant challenge
both in terms of labor intensiveness and scalability due to the large size and diversity of medical
cases encountered in practice.
This project harnesses the power of Generative Adversarial Networks (GANs), a potent class
of generative models, to address the challenge of manual annotation. By utilizing GANs, syn-
thetic pathological images are generated for training machine learning models across diverse digital
pathology applications. GANs, composed of a generator and discriminator trained adversarially,
progressively produce increasingly realistic data over time [1].
1. Train DatasetGAN with Pathological Images from https://bcsegmentation.grand-challeng
org/ : Implement and train the DatasetGAN framework using a curated set of pathologi-
cal images as input, as demonstrated in previous works of [2, 3, 4]. https://github.com/
SyedA5688/HistopathologyDatasetGAN
2. Adapt GAN to Enhance Pathological Tissue: Customize the generative adversarial
network (GAN) architecture to enhance the quality and realism of pathological tissue images,
leveraging techniques such as image-to-image translation [5].
3. Generate Synthetic Dataset: Utilize the adapted GAN to generate a synthetic dataset of
pathological images, including pixel-level annotations, that closely mimics real-world cases in
terms of pathology variations and image quality.
4. Train Segmentation Model: Develop a state-of-the-art segmentation model and train it
exclusively on the synthetic dataset generated in the previous step, aiming for accurate and
precise segmentation of pathological structures.
5. Evaluate Performance Against Full Supervision: Evaluate the performance of the seg-
mentation model on real-world pathological images and compare it against models trained
with full manual supervision, assessing segmentation accuracy, precision, recall, and F1-score.
Expected Outcomes
• A GAN-based generative model capable of creating extensive pathological image datasets,
complete with corresponding masks.
• A synthetic dataset featuring pixel-level annotations closely resembling those found in genuine
pathological images.
• Competently trained machine learning models adept at digital pathology tasks.
• A comprehensive understanding of the advantages and limitations associated with using ex-
clusively synthetic data for training purposes.
Benefits
• Alleviates the issue of limited data availability in digital pathology.
• Diminishes the need for extensive manual annotation efforts.
• Offers a viable avenue for expanding digital pathology research and applications through the
utilization of GAN-generated synthetic data.
[1] M. E. Tschuchnig, G. J. Oostingh, and M. Gadermayr, “Generative adversarial networks in
digital pathology: A survey on trends and future potential,” Patterns, vol. 1, p. 100089, 9 2020.
[2] Y. Zhang, H. Ling, J. Gao, K. Yin, J.-F. Lafleche, A. Barriuso, A. Torralba, and S. Fidler,
“Datasetgan: Efficient labeled data factory with minimal human effort.”
[3] S. A. Rizvi, P. Cicalese, S. V. Seshan, S. Sciascia, J. U. Becker, and H. V. Nguyen, “Histopathol-
ogy datasetgan: Synthesizing large-resolution histopathology datasets.”
[4] B. Lutnick and P. Sarder, “Generative modeling of histology tissue reduces human
annotation effort for segmentation model development.” [Online]. Available: https:
//doi.org/10.1101/2021.10.15.464564
[5] S. Butte, H. Wang, M. Xian, and A. Vakanski, “Sharp-gan: Sharpness loss regularized gan for
histopathology image synthesis.”
[6] J. Gui, Z. Sun, Y. Wen, D. Tao, and J. Ye, “A review on generative adversarial networks:
Algorithms, theory, and applications,” vol. 14, 2015.
[7] V. L. T. de Souza, B. A. D. Marques, H. C. Batagelo, and J. P. Gois, “A review on generative
adversarial networks for image generation,” Computers Graphics, vol. 114, pp. 13–25, 8 2023.
[8] “Github - syeda5688/histopathologydatasetgan.” [Online]. Available: https://github.com/
SyedA5688/HistopathologyDatasetGAN
[9] M. Han, H. Zheng, C. Wang, Y. Luo, H. Hu, and B. Du, “Leveraging gan priors for few-shot
part segmentation,” Proceedings of the 30th ACM International Conference on Multimedia, pp.
1339–1347, 10 2022.
[10] L. Jose, S. Liu, C. Russo, A. Nadort, and A. D. Ieva, “Generative adver-
sarial networks in digital pathology and histopathological image processing: A
review,” Journal of Pathology Informatics, vol. 12, p. 43, 1 2021. [Online].
Available: /pmc/articles/PMC8609288//pmc/articles/PMC8609288/?report=abstracthttps:
//www.ncbi.nlm.nih.gov/pmc/articles/PMC8609288/
[11] A. Xu, M. I. Vasileva, A. Dave, and A. Seshadri, “Handsoff: Labeled dataset generation with
no additional human annotations.”
[12] A. Manerikar and A. C. Kak, “Self-supervised one-shot learning for for automatic segmentation
of stylegan images.”